Decide if facts relating to nonconforming product and high quality problems and corrective and preventive actions is effectively disseminated, including dissemination for management critique.
So far Now we have carried out Document and Training Administration and also CAPA and both of those have every thing we'd like appropriate out in the box. Immediately after several years of cumbersome spreadsheets and databases CQ can be a blessing. It makes document administration swift and simple… and it’s a satisfaction to utilize. Helen Cary,
It concentrates on the systematic investigation of the foundation results in of identified difficulties or discovered pitfalls within an try and stop their recurrence (for corrective action) or to prevent incidence (for preventive action).
nine. Confirm that corrective and preventive actions for product and excellent troubles ended up executed and documented.
have an impact on the management procedure, but in which you are uncertain of the end result. This way of wondering entails figuring out this uncertainty, or danger, and identifying if you need to get action to forestall undesirable results or to capitalize on opportunities — primarily optimistic hazard.
Proof of efficiency shall be planned, done, and documented for all CAPAs. Evidence could possibly be shown by carrying out a verification/validation of your improved procedure, by monitoring the method in excess of an prolonged timeframe in accordance with the accredited acceptance conditions for success or by other appropriate means.
Rapid Containment: If the challenge poses a right away menace, just take containment actions to stop further difficulties. This might contain isolating impacted goods or products and services.
CAPA is just not basically a reactive corrective and preventive action example procedure; it is a proactive approach to excellent management that encompasses equally corrective and preventive measures.
Any deviation or difficulty can have evident causes and root leads to. Firms frequently tackle the plain causes. Although this may feel helpful from the short-term, the situation may perhaps persist or lead to unexpected consequences.
Although corrective and preventive action are each critical features in quality management, they provide different purposes. You may imagine corrective action as reactive, correcting
Our related suite of options allows enterprises of all measurements maximize product or service, top quality, security, and provider as they bring about their products from strategy to consumer achievements. Meet up with the Leadership Group
, but they are most commonly connected to ISO 9001. This Global common has the specs for applying and retaining a
Normally the foundation explanation for a root cause may be the method or not enough procedures, methods or procedures which supported the development with the Bodily root trigger. Preventive Action (PA) takes place after the Actual physical root bring about has actually been discovered and long lasting corrective action has become validated.
six) Set your plan set up. This is as simple as subsequent by way of on here the plan and rendering it materialize. It may be as straightforward as implementing the preventive maintenance system previously described, or obtaining and setting up a completely new piece of kit as the aged a person could now not keep the precision you need.
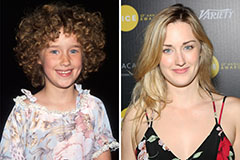 Ashley Johnson Then & Now!
Ashley Johnson Then & Now! Marla Sokoloff Then & Now!
Marla Sokoloff Then & Now!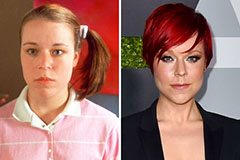 Tina Majorino Then & Now!
Tina Majorino Then & Now! Samantha Fox Then & Now!
Samantha Fox Then & Now! Bill Murray Then & Now!
Bill Murray Then & Now!